Hello Whyvillians! One of the courses I'm taking this semester is science. Surprisinglu, it's pretty fun. We've finished our chemistry unit, and now we're on the biology. In my opinion, biology isn't that interesting, but chemistry sure was! One of things we studied in chemistry was the periodic table of elements, which is what I'm going to talk to you about today. Wait! Don't leave yet! The periodic table isn't as boring as you may think it is . . .
That is the periodic table! Isn't it cool? I know what you're thinking . . . zzzzzz, but don't think that! I promise that the periodic table isn't a lame as you probably think it is.
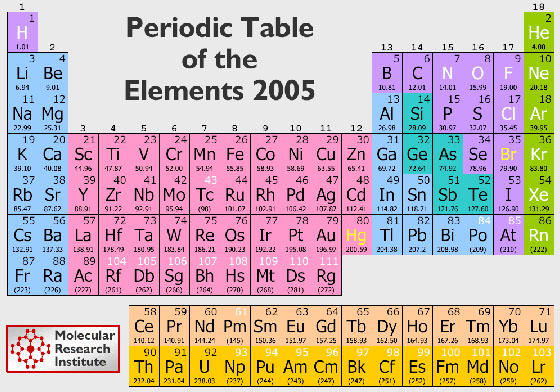
So what is this thing? A very good question. The periodic table organizes all of the known elements into families and periods. The families run vertically, and the periods horizontally. The periodic table was first invented in 1869, by Dmitri Mendeleev. Mendeleev was a teacher, who was trying to come up with a way to teacher his students about elements. He wrote each of the elements on a card, along with their atomic number. He then started arranging them, similar to a game of solitaire. He finally came up with the first edition of the periodic table. Since then, many more elements have been discovered. The picture above is the modern periodic table that we use today. It still follows Mendeleev's basic idea of arranging the elements into groups and periods, and using atomic numbers.
Now you know what it is, so you're probably wondering how it's arranged. First of all, if you look at the periodic table above, you'll see that instead of the elements' names, you find two (or in some cases 1) letters. Each element has their own symbol! The first letter is always capitalized, and the second always in lower case. The symbols for the elements are the same in every language. Some elements, like helium, have pretty straightforward symbols. He = Helium. However, some elements have their symbol after their Latin name, like lead. The Latin name for lead is plumbum, so Pb = Lead! Cool eh?
Each element has their own special little box that looks like this:

In it you see the element's symbol, atomic number, and atomic mass. Helium is number 2, and it's mass is 4.
So now you know who invented the periodic table, and what everything in each box stands for. I bet you'd like to know how they're grouped now! Let's start with the easiest group, the Noble Gases.
The Noble Gases
Do you see the lime green column on the periodic table above? This group of elements are the Noble Gases. Why are they called the noble gases? Well, all you need to know is that these gases won't react with anything else, so they are HAPPY! If you were a noble, you'd be happy, right? I'll talk about reactions a little later on. Helium, Neon, Argon, Krypton, Xenon and Radon make up this group. Where have you heard these names before? Splitzer Spectroscopy of course! You can see the spectrum of almost all the noble gases at the Splitzer Spectroscopy game.
The Alkali Metals
The Alkali Metals are the elements on the very far left, so group 1. Do you see how Hydrogen, H, is at the top of this group? Hydrogen doesn't really fit nicely anywhere in the periodic table, so it's just kind of stuck on the Alkali Metals (which is funny, because Hydrogen is a gas!). So technically, the first element of group 1 is Lithium Li. What is so cool about this group is how explosive they are! Yup, you heard me, explosive. These metals (Lithium, Sodium, Potassium, Rubidium, Cesium and Francium) are highly reactive to water! If you drop a tiny piece of Lithium into a bowl of water, it will fizz and zoom around the bowl. If you drop a tiny piece of Sodium into a bowl of water, it will fizz louder and zoom around the bowl quicker. If you drop a piece of Potassium into a bowl of water, the potassium will catch fire. Do you see the pattern? As you move down the group, each element's reaction with water is more severe. By the time you get to Cesium, the reaction is so great that the entire bowl with shatter from the explosion that occurs. And if that's what Cesium can do, imagine Francium!
Do you see all of the purple (and lime green) elements? They are all gases at room temperature (except for Br, that's a liquid!). Notice how all the gases are on the right side of the periodic table? I bet you've heard of some of them: C = Carbon, O = Oxygen, Cl = Chlorine. Br (Bromine) and Hg (Mercury) are the only two elements that are liquids at room temperature. The rest of the elements are all solids.
Remember how I was talking about atomic numbers before? Well, in case you were wondering, the atomic number is equal to the number of protons an element has in one of its atoms. If that's confusing, don't worry about it! You won't even have to learn this stuff until grade 9! Here's some other confusing stuff for you. The number of neutrons an element has is its atomic mass, minus its atomic number! If you'd like me to go more in-depth with this, post a thread in the BBS.
Finally, I'd like to talk about why the periodic table of elements is important. Having a good understanding of elements is very important in chemistry! Take the Alkali Metals for example. We know that as they descend, they become more and more reactive. Knowing this, would you really want to make something like a dental filling out of Sodium? Of course not! Your whole mouth could explode! However, based on your knowledge of the Noble Gases, you know that they are nonreactive. This means that it's pretty safe to inhale a bit of Helium out of a balloon!
The periodic table has many uses, and it's very important in science. Chances are, you'll be learning about it sometime in science class! When you do, be sure to refer back to this article, then you'll be ahead of the class! If you have any questions, post 'em in the BBS!
--rochrox
Author's Note: Sources: Science Power 9, Bill Nye the Science Guy: 100 Greatest Discoveries in Chemistry (Video)