I received an email today with a question about why water responds differently to the cold than other molecules or compounds. It's a great question, so I thought I'd share:
If cold air molecules move slower and take up less space than hot air molecules, why does water expand when it becomes ice? -Andrea
Neat! That's one of my favorite questions, since we always talk about things expanding when warm and contracting when cool - think about the air in your tires, or metal, just to name two. But one of the most obvious examples of a molecule that we see everyday - water - violates the rule. Sort of . . . Here's a summary of why water is so odd:
You may already know that water is a molecule made up of two hydrogen atoms bound to one oxygen atom. Water actually does contract when it cools down, just like most molecules do, but only until just above freezing temperature. Any colder than that, and the molecules become fixed into a very organized 3-dimensional pattern called a lattice. The lattice is formed because of super-strong hydrogen bonds among the water molecules, due to the way the electrons are organized around the atoms. So, the Hydrogen from one molecule of H2O interacts with the Oxygen from a neighboring one, and so forth.
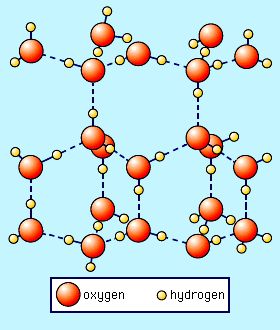
The dotted lines in this image represent the hydrogen bonds.
The rigid pattern leaves a lot of extra space in between the molecules, even more space than when the molecules were liquid. So by the time ice freezes, it's about 10% larger than when it was liquid. The extra space also makes frozen water less dense than water, which is why your ice cubes float at the top of your glass. The cool thing is that these hydrogen bonds are formed all the time, even when water is liquid, but the low temperature forces the bonds into a very specific orientation, or structure, (which is really pretty!)
What do you think happens to ice once it's frozen? Will it expand even more? (hint: you might be able to test this at home . . .) Think about and let me know!
Author's Note: Sources:
http://www.chem1.com/acad/sci/aboutwater.html
http://www.its.caltech.edu/~atomic/snowcrystals/primer/primer.htm
Editor's Note: For more blogs from Dr. Rabiah, visit Science Chicago's website at: http://www.sciencechicagoblog.com